With global scientific breakthroughs and evolving regulatory landscapes, now is the crucial time to accelerate clinical development of cannabinoid therapeutics.
Returning for its 8th year, the summit offers an expanded program featuring a new business development and investor panel, dedicated intellectual property protection sessions, and interactive regulatory discussions to help you navigate the path to approval.
Alongside these new additions, you’ll also discover the pivotal milestones and hard-earned clinical development lessons from the past 12 months, shared by the pioneering biotechs driving progress in the field.
You Can Look Forward To:
Strategic Investment & Business Development Insights:
Join a dedicated panel of investors and industry executives to discover what makes cannabinoid programs attractive for funding. Learn how to structure partnerships and position your pipeline for long-term commercial success.
Advanced Formulation Strategies:
Delve into cutting-edge formulation techniques, including innovative delivery systems and optimized formulations designed to improve bioavailability, enhance stability, and support patient adherence in clinical settings.
Expert Guidance on IP Protection:
Join leading cannabinoid IP lawyers Chris Hayes and Graham Penchik for expert guidance on developing a robust patent strategy. Learn how to safeguard your innovations from early discovery through to commercialization
Regulatory Pathway Navigation:
Hear directly from regulatory experts as they unpack the complexities of global approval pathways for cannabinoid-based therapeutics. Gain actionable insights to overcome regulatory hurdles and accelerate approvals.
Clinical Development Lessons & Milestones:
Access exclusive case studies from pioneering biotechs sharing critical learnings from recent clinical trials. Take away practical strategies to address trial design challenges, placebo control, and achieving regulatory acceptance.
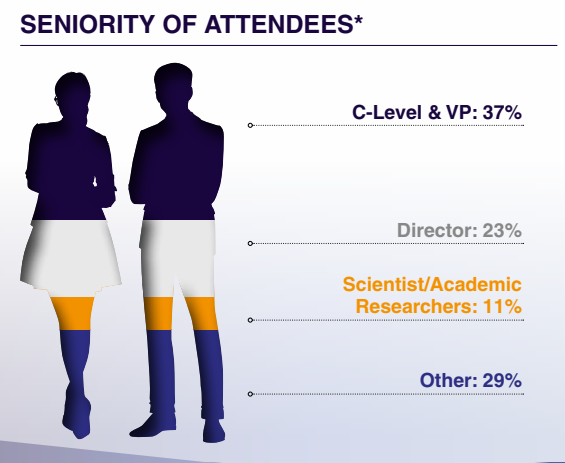
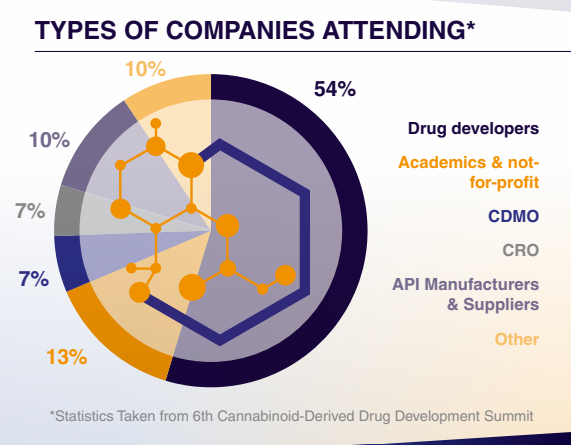
Who Will You Meet?
This summit connects C-level suite, vice presidents, directors and senior scientists who specialise in pre-clinical and clinical development, pharmacology, translational science and regulatory affairs.
What Your Peers Have to Say



